Find the Ph of a 0.330 M Nac2h3o2 Solution
Chemical compound
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name Sodium acetate | |
| Other names Hot ice (sodium acetate trihydrate) | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) |
|
| Beilstein Reference | 3595639 |
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| DrugBank |
|
| ECHA InfoCard | 100.004.386 |
| EC Number |
|
| E number | E262 (preservatives) |
| Gmelin Reference | 20502 |
| KEGG |
|
| PubChem CID |
|
| RTECS number |
|
| UNII |
|
| CompTox Dashboard (EPA) |
|
| InChI
| |
| SMILES
| |
| Properties | |
| Chemical formula | C 2 H 3 Na O 2 |
| Molar mass | 82.034 g·mol−1 |
| Appearance | White deliquescent powder |
| Odor | Vinegar (acetic acid) odor when heated to decomposition[1] |
| Density | 1.528 g/cm3 (20 °C, anhydrous) 1.45 g/cm3 (20 °C, trihydrate)[2] |
| Melting point | 324 °C (615 °F; 597 K) (anhydrous) 58 °C (136 °F; 331 K) (trihydrate) |
| Boiling point | 881.4 °C (1,618.5 °F; 1,154.5 K) (anhydrous) 122 °C (252 °F; 395 K) (trihydrate) decomposes |
| Solubility in water | Anhydrous: 119 g/100 mL (0 °C) 123.3 g/100 mL (20 °C) 125.5 g/100 mL (30 °C) 137.2 g/100 mL (60 °C) 162.9 g/100 mL (100 °C) Trihydrate: 32.9 g/100 mL (-10 °C) 36.2 g/100 mL (0 °C) 46.4 g/100 mL (20 °C) 82 g/100 mL (50 °C)[3] |
| Solubility | Soluble in alcohol, hydrazine, SO2 [4] |
| Solubility in methanol | 16 g/100 g (15 °C) 16.55 g/100 g (67.7 °C)[4] |
| Solubility in ethanol | Trihydrate: 5.3 g/100 mL |
| Solubility in acetone | 0.5 g/kg (15 °C)[4] |
| Acidity (pK a) | 24 (20 °C)[4] 4.75 (when mixed with CH3COOH as a buffer)[5] |
| Basicity (pK b) | 9.25 |
| Magnetic susceptibility (χ) | −37.6·10−6 cm3/mol |
| Refractive index (n D) | 1.464 |
| Structure | |
| Crystal structure | Monoclinic |
| Thermochemistry | |
| Heat capacity (C) | 100.83 J/mol·K (anhydrous)[6] 229 J/mol·K (trihydrate)[7] |
| Std molar | 138.1 J/mol·K (anhydrous)[6] 262 J/mol·K (trihydrate)[2] |
| Std enthalpy of | −709.32 kJ/mol (anhydrous)[4] −1604 kJ/mol (trihydrate)[2] |
| Gibbs free energy (Δf G˚) | −607.7 kJ/mol (anhydrous)[4] |
| Pharmacology | |
| ATC code | B05XA08 (WHO) |
| Hazards | |
| Main hazards | Irritant |
| Safety data sheet | External MSDS |
| NFPA 704 (fire diamond) | 1 1 0 |
| Flash point | >250 °C (482 °F; 523 K) [5] |
| Autoignition | 600 °C (1,112 °F; 873 K)[5] |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 3530 mg/kg (oral, rat) |
| Related compounds | |
| Other anions | Sodium formate Sodium propionate |
| Other cations | Potassium acetate Calcium acetate |
| Related compounds | Sodium diacetate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Chemical compound
Sodium acetate, NaCH3COO, also abbreviated NaOAc,[8] is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses.
Applications [edit]
Biotechnological [edit]
Sodium acetate is used as the carbon source for culturing bacteria. Sodium acetate is also useful for increasing yields of DNA isolation by ethanol precipitation.
Industrial [edit]
Sodium acetate is used in the textile industry to neutralize sulfuric acid waste streams and also as a photoresist while using aniline dyes. It is also a pickling agent in chrome tanning and helps to impede vulcanization of chloroprene in synthetic rubber production. In processing cotton for disposable cotton pads, sodium acetate is used to eliminate the buildup of static electricity.
Concrete longevity [edit]
Sodium acetate is used to mitigate water damage to concrete by acting as a concrete sealant, while also being environmentally benign and cheaper than the commonly used epoxy alternative for sealing concrete against water permeation.[9]
Food [edit]
Sodium acetate may be added to food as a seasoning, sometimes in the form of sodium diacetate, a one-to-one complex of sodium acetate and acetic acid,[10] given the E-number E262. It is often used to give potato chips a salt and vinegar flavour, and may be used as a substitute for vinegar itself on potato chips as it doesn't add moisture to the final product.[11] Sodium acetate (anhydrous) is widely used as a shelf-life extending agent, pH control agent[12] It is safe to eat at low concentration.[13]
Buffer solution [edit]
A solution of sodium acetate (a basic salt of acetic acid) and acetic acid can act as a buffer to keep a relatively constant pH level. This is useful especially in biochemical applications where reactions are pH-dependent in a mildly acidic range (pH 4–6).
Heating pad [edit]

Sodium acetate is also used in heating pads, hand warmers, and hot ice. Sodium acetate trihydrate crystals melt at 136.4 °F/58 °C[14] (to 137.12 °F/58.4 °C),[15] dissolving in their water of crystallization. When they are heated past the melting point and subsequently allowed to cool, the aqueous solution becomes supersaturated. This solution is capable of cooling to room temperature without forming crystals. By pressing on a metal disc within the heating pad, a nucleation center is formed, causing the solution to crystallize back into solid sodium acetate trihydrate. The bond-forming process of crystallization is exothermic.[16] The latent heat of fusion is about 264–289 kJ/kg.[14] Unlike some types of heat packs, such as those dependent upon irreversible chemical reactions, a sodium acetate heat pack can be easily reused by immersing the pack in boiling water for a few minutes, until the crystals are completely dissolved, and allowing the pack to slowly cool to room temperature.[17]
Preparation [edit]

A crystal of sodium acetate trihydrate (length 1.7 centimetres)
For laboratory use, sodium acetate is inexpensive and usually purchased instead of being synthesized. It is sometimes produced in a laboratory experiment by the reaction of acetic acid, commonly in the 5–8% solution known as vinegar, with sodium carbonate ("washing soda"), sodium bicarbonate ("baking soda"), or sodium hydroxide ("lye", or "caustic soda"). Any of these reactions produce sodium acetate and water. When a sodium and carbonate ion-containing compound is used as the reactant, the carbonate anion from sodium bicarbonate or carbonate, reacts with hydrogen from the carboxyl group (-COOH) in acetic acid, forming carbonic acid. Carbonic acid readily decomposes under normal conditions into gaseous carbon dioxide and water. This is the reaction taking place in the well-known "volcano" that occurs when the household products, baking soda and vinegar, are combined.
- CH3COOH + NaHCO3 → CH3COONa + H2CO
3 - H2CO
3 → CO
2 + H
2 O
Industrially, sodium acetate trihydrate is prepared by reacting acetic acid with sodium hydroxide using water as the solvent.
- CH3COOH + NaOH → CH3COONa + H2O
Structure [edit]
The crystal structure of anhydrous sodium acetate has been described as alternating sodium-carboxylate and methyl group layers.[18] Sodium acetate trihydrate's structure consists of distorted octahedral coordination at sodium. Adjacent octahedra share edges to form one-dimensional chains. Hydrogen bonding in two dimensions between acetate ions and water of hydration links the chains into a three-dimensional network.[19] [20]
| Degree of hydration | Na coordination | Strongly bonded aggregation | Weakly bonded aggregation |
|---|---|---|---|
| Anhydrous[18] | 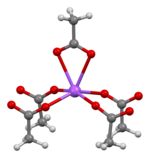 |  2D sheet |  sheets stacked with hydrophobic surfaces in contact |
| Trihydrate[19] [20] |  |  1D chain |  chains linked by hydrogen bonds (one chain highlighted in light blue) |
Reactions [edit]
Sodium acetate can be used to form an ester with an alkyl halide such as bromoethane:
- CH3COONa + BrCH2CH3 → CH3COOCH2CH3 + NaBr
Sodium acetate undergoes decarboxylation to form methane (CH4) under forcing conditions (pyrolysis in the presence of sodium hydroxide):
- CH3COONa + NaOH → CH4 + Na2CO3
Calcium oxide is the typical catalyst used for this reaction. Caesium salts also catalyze this reaction.[ citation needed ]
References [edit]
- ^ "Sodium Acetate". International Chemical Safety Cards. National Institute of Occupational Safety and Health. 2018-09-18.
- ^ a b c "sodium acetate trihydrate". chemister.ru.
- ^ Seidell, Atherton; Linke, William F. (1952). Solubilities of Inorganic and Organic Compounds. Van Nostrand.
- ^ a b c d e f "sodium acetate". chemister.ru.
- ^ a b c Sigma-Aldrich Co., Sodium acetate. Retrieved on 2014-06-07.
- ^ a b Acetic acid, sodium salt in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD), http://webbook.nist.gov (retrieved 2014-05-25)
- ^ Acetic acid, sodium salt, hydrate (1:1:3) in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD), http://webbook.nist.gov (retrieved 2014-05-25)
- ^ Clayden, Jonathan; Greeves, Nick; Warren, Stuart; Wothers, Peter (2001). Organic Chemistry (1st ed.). Oxford University Press. ISBN978-0-19-850346-0.
- ^ "Potato Chip Flavoring Boosts Longevity Of Concrete". Science Daily. 8 August 2007.
- ^ AG, Jungbunzlauer Suisse. "Sodium Diacetate – Jungbunzlauer". www.jungbunzlauer.com.
- ^ Austen, Ian (2018-06-09). "The Secret Story of Salt and Vinegar Chips: the Canada Letter". The New York Times. ISSN 0362-4331. Retrieved 2021-11-23 .
- ^ "Food Additive "Sodium Acetate (Anhydrous)" | Products". Mitsubishi Chemical Corporation . Retrieved 16 September 2020.
- ^ Mohammadzadeh-Aghdash, Hossein; Sohrabi, Yousef; Mohammadi, Ali; Shanehbandi, Dariush; Dehghan, Parvin; Ezzati Nazhad Dolatabadi, Jafar (15 August 2018). "Safety assessment of sodium acetate, sodium diacetate and potassium sorbate food additives". Food Chemistry. 257: 211–215. doi:10.1016/j.foodchem.2018.03.020. ISSN 0308-8146. PMID 29622200. Retrieved 16 September 2020.
- ^ a b Ibrahim Dincer and Marc A. Rosen. Thermal Energy Storage: Systems and Applications, page 155
- ^ Courty JM, Kierlik E, Les chaufferettes chimiques, Pour la Science, décembre 2008, pp. 108–110
- ^ "Crystallization of Supersaturated Sodium Acetate". Journal of Chemical Education. 2015-07-19.
- ^ "How do sodium acetate heat pads work?". HowStuffWorks. April 2000. Retrieved 2007-09-03 .
- ^ a b Hsu, Leh-Yeh; Nordman, C. E. (1983). "Structures of two forms of sodium acetate, Na+.C2H3O2 −". Acta Crystallogr. C. 39 (6): 690–694. doi:10.1107/S0108270183005946.
- ^ a b Cameron, T. S.; Mannan, K. M.; Rahman, M. O. (1976). "The crystal structure of sodium acetate trihydrate". Acta Crystallogr. B. 32: 87–90. doi:10.1107/S0567740876002367.
- ^ a b Wei, K.-T.; Ward, D. L. (1977). "Sodium acetate trihydrate: a redetermination". Acta Crystallogr. B. 33 (2): 522–526. doi:10.1107/S0567740877003975.
External links [edit]
- Hot Ice – Instructions, Pictures, and Videos
- How Sodium Acetate heating pads work
- Lavars, Nick (2021-09-15). "Sodium acetate acts as a potential fountain of youth for aging bones". New Atlas . Retrieved 2021-09-16 .
Find the Ph of a 0.330 M Nac2h3o2 Solution
Source: https://en.wikipedia.org/wiki/Sodium_acetate
